
For Polypropylene (PP)
General
Polypropylene (PP) in their natural state (without additives) are inherently unstable and degrade when exposed to oxygen. The polymers change color to yellow-brown and begin to flake away until the material becomes useless.
When PP degrades, chain scission takes place. The physical properties of the polymer deteriorate and its average molecular weight (chain length) decreases, melt flow rate increases and a powdery surface eventually forms. Polymer degradation is a natural phenomenon that cannot be totally stopped. Instead, resin producers seek to stabilize the color and physical properties of their polymers for a reasonable life span, which varies depending on the end use requirements.
Applications
Polypropylene can be processed by virtually all thermoplastic-processing methods. Most typically PP products are manufactured by Extrusion Blow Molding, Injection Molding, and General Purpose Extrusion Additives are needed to stabilize polypropylene during melt processing and protect plastics against thermo-oxidative degradation during service life:
Melt processing stability of polypropylene can be quantified by measuring molecular weights distribution, melt flow or viscosity, and discoloration before and after processing in an extruder for example.
As most polypropylene articles are exposed to oxygen, elevated temperatures, light, and moisture during their service lives, thermo-oxidation occurs. The thermo-oxidative stability of a PP plastic part during services is determined by aging the part at elevated temperatures in a circulating air oven for example. The property called Long Term Thermal Stability (LTTS) is measured. Mechanical properties such as embrittlement on bending, elongation, tensile impact are determined as a function of aging time.
For Polyethylene (PE)
Polyethylene (PE) are a known class of thermoplastic polymers having many members. They are prepared by homo-polymerizing ethylene or inter-polymerizing (e.g., co-polymerizing) ethylene. With one or more alpha-olefins having from 3 to about 18 carbon atoms by known polymerization reactions and conditions.
The viscosity of polyethylene which have pendant vinyl . And/or vinylidene groups tends to change during melt process operations, e.g during extrusion, molding, etc. Such thermally-induced changes in viscosity have been attributed to the changes in molecular weight. And/or linearity of the polymers caused by cross-linking.
A wide variety of “stabilizers” have been developed to reduce the changes (e.g., cross-linking) that can occur during melt processing or under conditions of use. Many of the stabilizers are organic compounds which are classified in the plastics industry as Fillplas‘s Antioxidant.
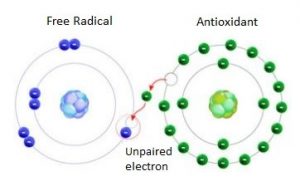
Many antioxidant tend to function as free radical scavengers and they interact with free radicals that are formed during polymerization or in the presence of air or other oxidizing medium.
Antioxidant are a known class of stabilizers which includes, for example, hindered phenols, triaryl phosphites, aromatic amines, hydroxylamines, and the like.



